Cardiac Action Potentials: From Depolarization to Myocardial Contraction
May 12, 2025While cardiac action potentials are often reduced to textbook waveforms and phases, their ultimate purpose is mechanical: to produce calcium influx that activates myocardial contraction. Every depolarization on an ECG—every P wave, QRS complex, and T wave—represents a deeper cellular orchestration of ion shifts culminating in calcium-mediated mechanical force.
In this post, we’ll dive into the physiology of the cardiac action potential, focusing on how calcium influx is the key bridge between electrical excitation and mechanical contraction. We'll also walk through the intracellular mechanisms that translate calcium entry into cross-bridge cycling and systolic ejection.
You can learn more about the anatomical basis of ECG in my course ECG in 21 Days.
The Cardiac Action Potential: Not Just an Electrical Event
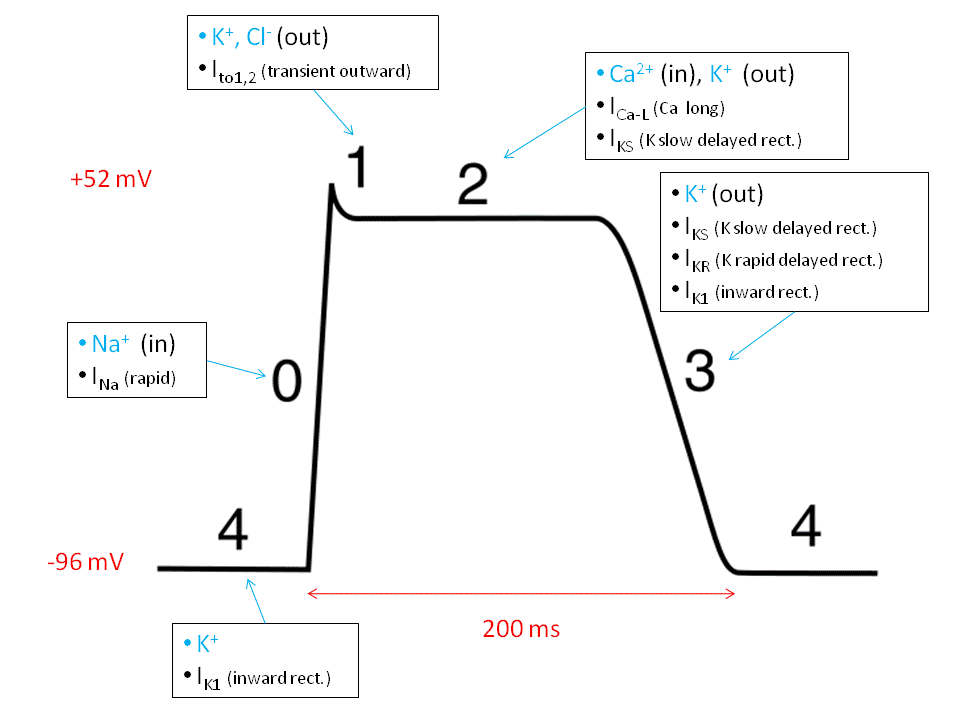
Cardiac action potentials vary by cell type—nodal cells vs. myocytes—but phase 0 to phase 3 are the classic stages in working ventricular myocytes:
-
Phase 0 (Depolarization): Rapid influx of Na⁺ through voltage-gated sodium channels.
-
Phase 1 (Initial repolarization): Transient outward K⁺ current.
-
Phase 2 (Plateau): Influx of Ca²⁺ through L-type calcium channels, balanced by K⁺ efflux.
-
Phase 3 (Repolarization): K⁺ efflux dominates; Ca²⁺ channels close.
-
Phase 4 (Resting potential): Restoration via Na⁺/K⁺ ATPase and ion gradients.
The plateau phase (Phase 2) is where electrical activity achieves its mechanical purpose.
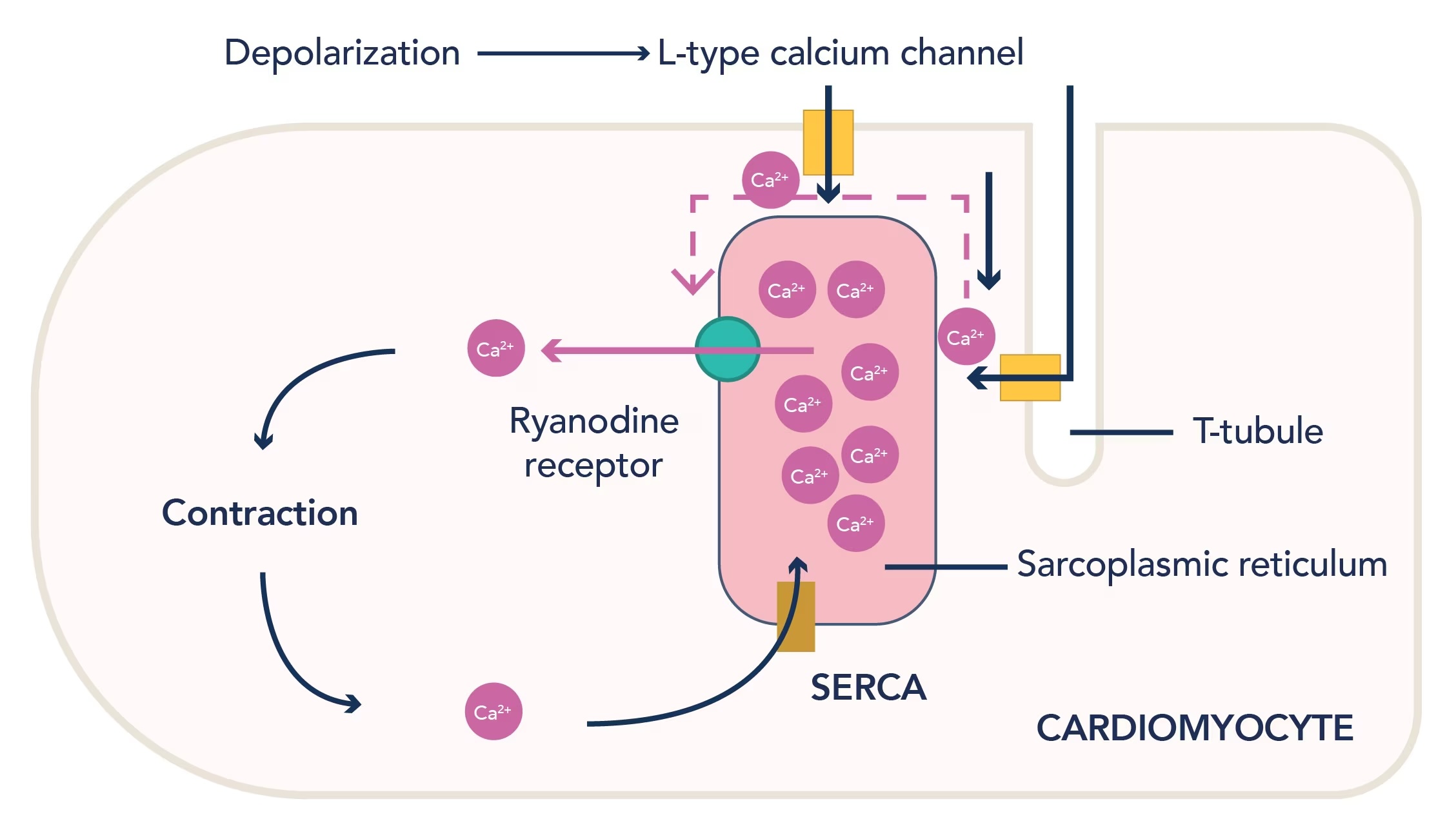
Calcium Influx: The Bridge Between Action Potential and Contraction
During Phase 2, L-type (long-lasting) calcium channels open in response to membrane depolarization. These channels allow a small but critically timed influx of extracellular Ca²⁺ into the cytosol.
But this initial calcium influx isn’t sufficient alone to generate contraction—it serves as a trigger.
Calcium-Induced Calcium Release (CICR)
-
The small amount of calcium entering through L-type channels binds to ryanodine receptors (RyR2) on the sarcoplasmic reticulum (SR).
-
This binding induces a massive release of stored calcium from the SR into the cytosol.
-
The rise in intracellular calcium concentration initiates contraction.
https://www.ptglab.com/news/blog/the-heart-of-the-matter-voltage-gated-calcium-channels-in-cardiovascular-disease/
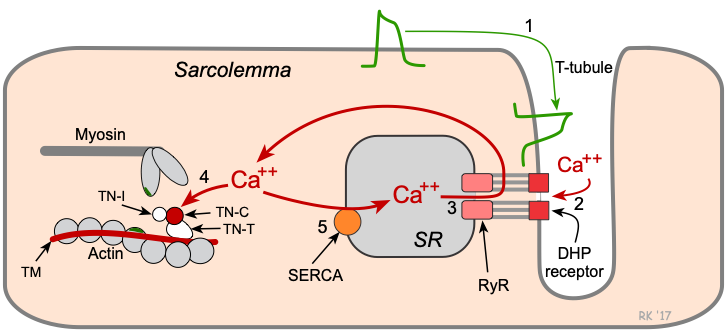
From Calcium to Contraction: The Sliding Filament Mechanism
-
Calcium binds troponin C on the thin filament.
-
This causes a conformational shift in tropomyosin, exposing myosin-binding sites on actin.
-
Myosin heads—primed with ATP—bind to actin and perform a power stroke, pulling the actin filament.
-
ATP is hydrolyzed, and the cycle repeats as long as intracellular calcium remains elevated.
This is the core of myocardial contraction: a coordinated, calcium-mediated mechanical event triggered by the electrical impulse seen on ECG.
https://cvphysiology.com/cardiac-function/cf022
Relaxation: Calcium Removal and Diastole
To terminate contraction, calcium must be cleared from the cytosol, which occurs via:
-
Reuptake into the SR via SERCA pumps (sarcoplasmic/endoplasmic reticulum Ca²⁺ ATPase).
-
Extrusion across the sarcolemma by:
-
Na⁺/Ca²⁺ exchanger (NCX).
-
Plasma membrane Ca²⁺ ATPase (PMCA).
-
The timing and efficiency of calcium removal are crucial to diastolic relaxation and ventricular filling.
Clinical Implications
-
Inotropes like digoxin and catecholamines enhance intracellular calcium availability and contractility.
-
Calcium channel blockers (e.g., verapamil, diltiazem) reduce calcium influx during phase 2, decreasing myocardial oxygen demand and contractile force.
-
Abnormalities in calcium handling underlie arrhythmias, heart failure, and diastolic dysfunction.
Take-Home Points
-
The end goal of depolarization in cardiac myocytes is calcium entry, not just voltage change.
-
L-type calcium channels initiate a cascade of calcium release from the sarcoplasmic reticulum, which activates contraction.
-
Calcium acts as a second messenger, linking membrane depolarization to actin-myosin cross-bridge cycling.
-
Relaxation is an active process requiring calcium clearance, critical to allow proper diastole.
Enjoy ECG Lectures with Reid? Here is a special gift from Reid
100 High Yield Annotated ECGs
Click below to download this free resource.